
Penguin Research Publications
SEABIRD MONITORING INSTRUCTION MANUAL

SEABIRD MONITORING INSTRUCTION MANUAL
Bellew L (2019) The Chilean island where the presence of cruise passengers is helping penguins breed. The Telegraph, 1st August 2019
Bingham M and Mejias E (1999) Penguins of the Magellan Region. Scientia Marina Vol:63, Supl. 1: 485-493
Bingham M (1999) Field Guide to Birds of the Falkland Islands.
Sponsored by The Darwin Initiative
CONTENTS
1. INTRODUCTION
1. INTRODUCTION
Magellanic penguins (Spheniscus magellanicus) are only found around southern South America, with breeding populations in Chile, Argentina and the Falkland Islands. Best guess estimates put the current world population of Magellanic penguins at around 1.5 million breeding pairs, with approximately 700,000 pairs in Chile, 650,000 pairs in Argentina and 150,000 pairs in the Falkland Islands (Bingham 1998, Bingham & Mejias 1999, Gandini et al. 1998).
Population studies in the Falkland Islands conducted by Dr Mike Bingham have revealed an 80% decline in Magellanic penguins between 1990/91 and 2002/03. The removal of fish and squid by large-scale commercial fishing vessels appears to be the cause of the Falklands decline, with high rates of chick and juvenile mortality from starvation leading to a lack of recruitment (Bingham 2002, Boersma 1997).
Population studies conducted in Argentina show evidence of decline at some colonies, but not all (Boersma 1997). Declines in Argentina appear to be largely the result of high adult and juvenile mortality caused by oil pollution. An estimated 40,000 Magellanic penguins are killed by oil pollution every year along the coast of Argentina, representing the main cause of adult mortality (Gandini et al. 1994). No population studies have yet been carried out on Magellanic penguins in Chile, even though Chile holds around half the world's population. The reason for this is lack of financial resources, which has not only prevented the establishment of a long-term monitoring programme, but also inhibited training of local personnel in seabird monitoring techniques.
One of Chile's largest and most important Magellanic penguin breeding sites is situated on Magdalena Island in the Straits of Magellan. Provisional examination suggests that Magellanic penguins are not declining on Magdalena Island, despite its close proximity to the Falklands (Bingham 2002, Bingham & Mejias 1999), but a long-term monitoring programme needs to be established in order to accurately determine population trends.
The island has been designated a national nature reserve because of its importance as a Magellanic penguin breeding site, and it is managed by the government agency Corporación Nacional Forestal (CONAF). The island is a popular tourist destination, so it is important to monitor the effects of tourism on penguin survival and breeding success, in order to ensure sustainable use of the reserve as a tourist resource. Magdalena Island holds a population of around 60,000 breeding pairs of Magellanic penguin, making it an ideal site at which to establish Chile's first long-term penguin monitoring programme.
2. BASELINE SURVEY
In order to correctly interpret the findings of any long-term monitoring programme on Magdalena Island, it was essential to conduct an Environmental Baseline Survey of the island. An Environmental Baseline Survey aims to provide the best practicable assessment of the abundance and distribution of birds and mammals, and to map out the vegetation and habitat types which support them. This provides baseline data with which to assess future changes in any component of the island's ecosystem.
2A. HABITAT
The first step of a conventional baseline survey is to identify and map out the key vegetation/habitat types found within the study area (Hiscock 1993). Initial studies undertaken by Dr Bingham identified the key vegetation/habitat types occurring in the region, including those which are not found on Magdalena itself (Appendices 1 & 2).
A survey of Magdalena Island was then conducted to map out the location and area of each vegetation/habitat type present on the island. This was performed by walking the entire coastline of the island, once along the littoral zone, and once along the adjacent terrestrial zone. The island was also repeatedly traversed in order to ensure that the interior was mapped out correctly according to the vegetation/habitat types present.
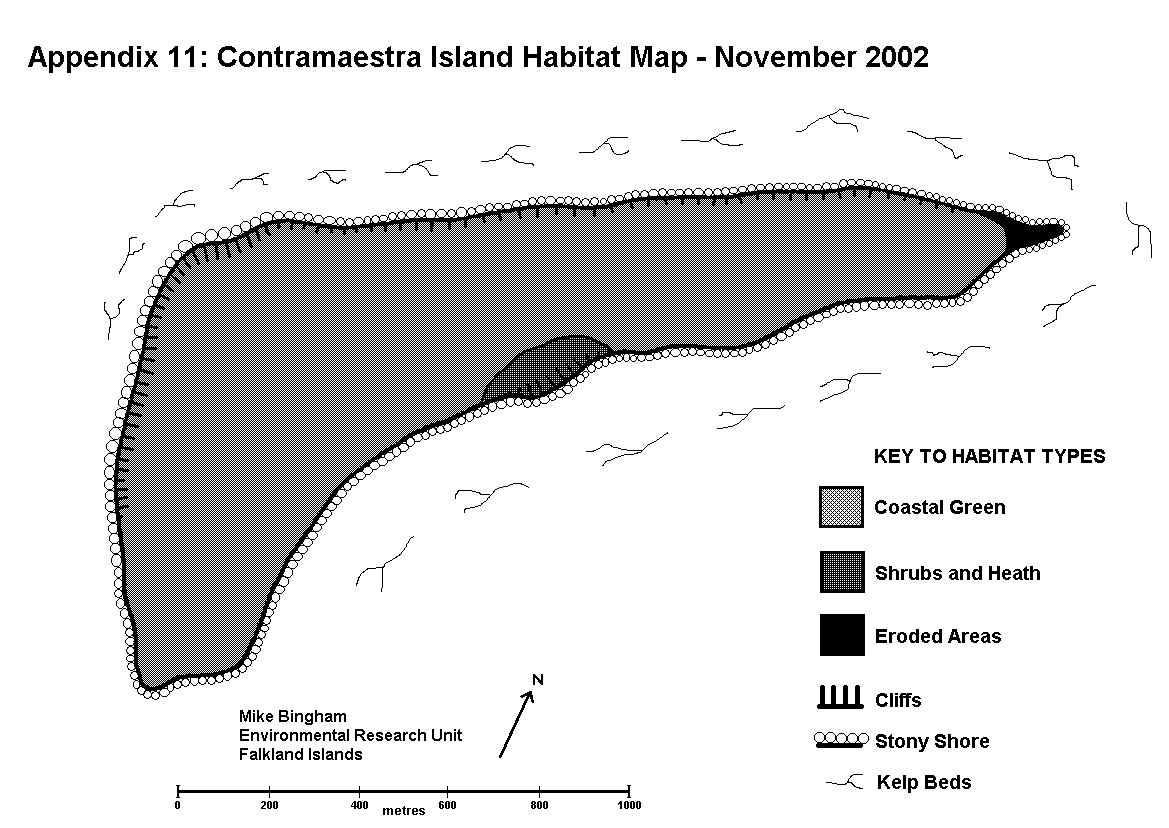
The littoral and terrestrial vegetation/habitat types were mapped out on field maps during the survey, and later copied onto the final survey map (Appendix 3). This method is consistent with MNCR/NCC Phase 1 Survey methodology (Nature Conservancy Council CSD Report No.1072 / Marine Nature Conservation Review Occasional Report MNCR/OR/05). The results will allow future changes in vegetation and habitat to be recorded, in order to observe potential links between changes in fauna and their associated habitat.
2B. FAUNA
A baseline survey of all birds and mammals present on the island was also recorded. Birds and mammals which breed in colonies can be accurately recorded by counting the number of breeding pairs in each colony, and mapping the colony locations. Species which breed individually require different techniques, depending on whether they are coastal birds or inland birds. Magellanic penguins are loosely colonial, breeding in burrows over a large area. Small Magellanic penguin colonies can be counted as per colonial birds, but larger colonies, such as found on Magdalena Island, require measurements of nesting density and area to determine total population size.
3. POPULATION CENSUS
3A. COLONIAL BIRDS & MAMMALS
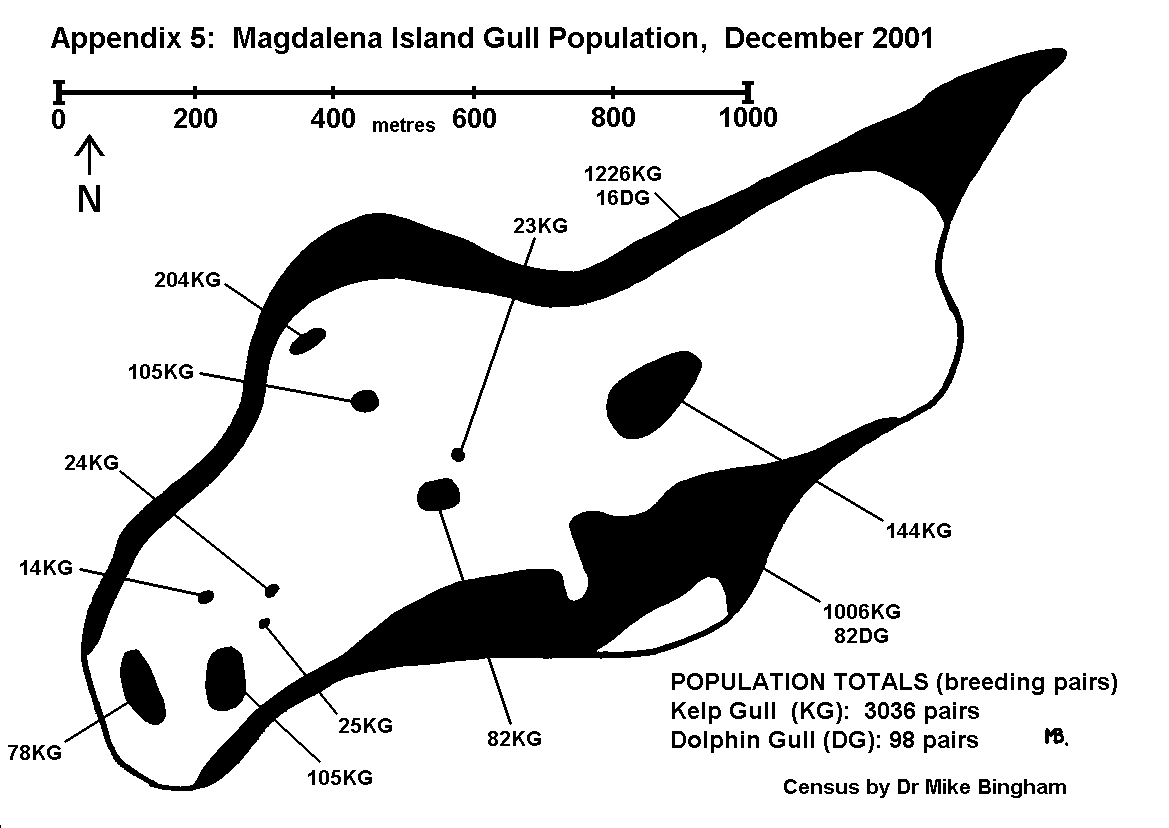
During an initial survey of the study area, all breeding colonies of birds and mammals were located and recorded on the map using a letter code (Appendix 4). These colonies were then visited at the appropriate stage of the breeding cycle to record the number of breeding pairs within each colony.
Counts are generally expressed in terms of breeding pairs, since this is the only meaningful figure for measuring population size. The number of individuals present within a colony will change during the course of the day, as individuals come and go in order to feed. The number of breeding pairs provides a constant measure of colony size regardless of daily changes.
For bird colonies, population counts are taken at the end of the egg-laying period, when incubation of the eggs has just begun. Counts are made of occupied nests only, which equates to the number of breeding pairs. Only incubating birds that are lying or sitting on nests are counted. Birds which are not on nests are ignored, since they are either non-breeders, or have partners nearby that are on nests. If two birds occupy the same nest only one is counted.
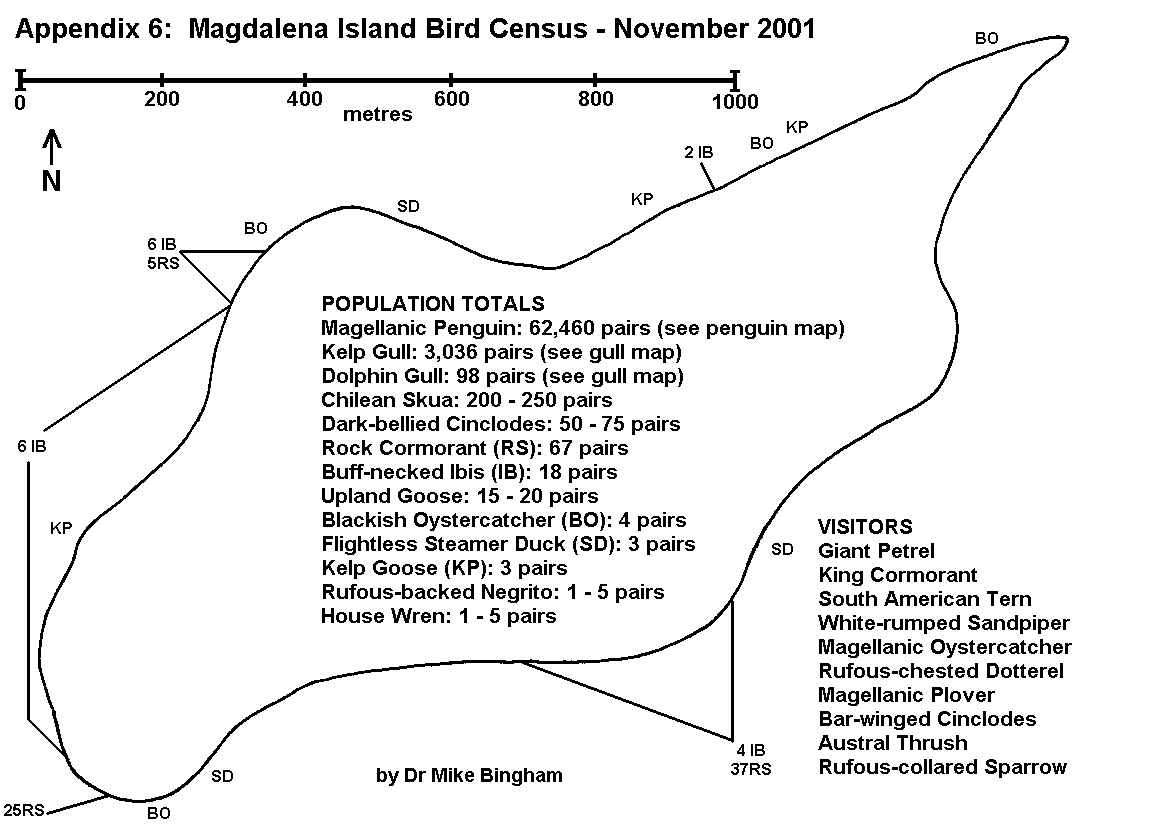
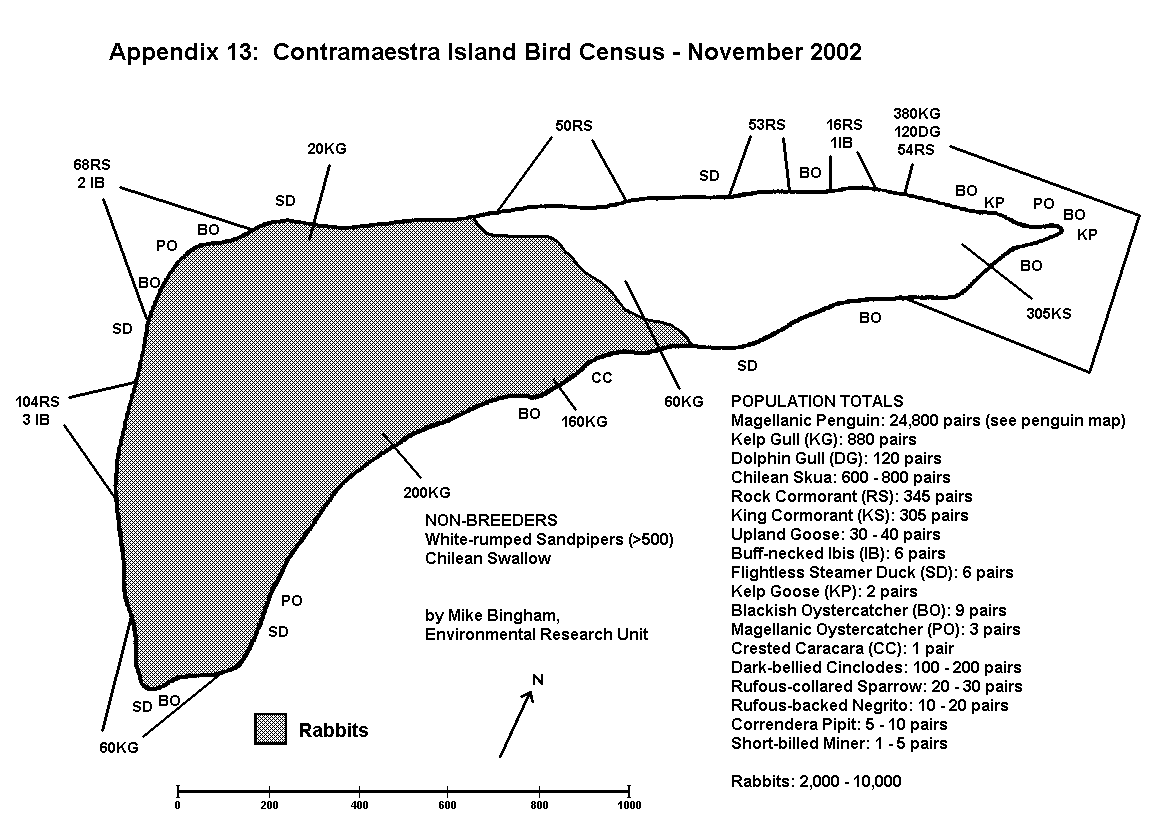
By conducting counts at the end of the egg-laying period, under-estimates of population resulting from abandoned or failed nests are kept to a minimum. Counts are recorded using tally-counters, with three nest counts being taken at each colony. The result is the mean of the three counts, whilst the spread of results gives an indication of the margin of error. For small discrete colonies the margin of error can be well below plus or minus 5%, but a margin of error of plus or minus 10% is usually allowed for counts of this type. The number of breeding pairs within each colony is entered on the map, along with the letter code indicating the species, and an arrow pointing to the exact location of the colony (Appendix 5 and 6).
The only colonial mammals likely to be encountered on Magdalena Island are pinipeds (seals & sealions). Pinipeds do not have nests, and dominant males often mate with several females, so breeding females are the nearest equivalent to breeding pairs. Since it is not possible to be certain which females have mated, population counts rely on counting pups. This is not ideal, since it only records successful births, but it is the internationally accepted method of determining population size for pinipeds. Counts are made upon completion of pup births, although some under-estimation is inevitable due to pup loses prior to counting, or late births. Nevertheless with careful timing of the census the margin for error should be within plus or minus 10%. Counts are recorded on the map as per colonial birds.
On Magdalena Island, gulls (Appendix 5) and cormorants (Appendix 6) were the only colonial birds recorded (excluding Magellanic penguins which are semi-colonial and covered separately). No pinipeds were recorded breeding on Magdalena Island.
3B. NON-COLONIAL BIRDS
SHOREBIRDS
Shorebirds, such as oystercatchers, marine ducks and marine geese, nest above the high water mark and patrol a territory that includes a section of beach. Because their breeding territories are restricted to the coastal strip, population size can be determined by walking the coastline. This is aided by the fact that such species are territorial and conspicuous, with the male usually holding a prominent position overlooking his territory.
During the incubation phase at least one bird from each pair (usually the female) will be sitting on eggs and well hidden from sight, increasing the likelihood of missing the pair if the male is resting. Once the chicks have hatched, they generally leave the nest and forage along the littoral and sub-littoral zones under the supervision of the adults, making the pair very visible and easy to count. Shorebird census work is therefore best conducted after the chicks have hatched, although the timing of the census is not as critical as for colonial birds.
Pairs that fail to breed will remain as a pair within their territory where they can still be visible for counting, so population size will not be underestimated as a result of failed breeders, as would be the case for colonial birds. Margins of error associated with shorebird counts are usually very low, although some error may arise when determining the breeding status of single birds encountered along the shore.
Counts are made of breeding pairs rather than individuals, but when counting shorebirds it is common to see only one member of the pair. A male that is prominently positioned, or which calls and shows alarm when approached, will probably have a female close by and should be counted. Lone females, or males that leave the area when approached, are probably non-breeders and should not be counted. A repeat census two or three weeks later will help to determine the status of lone birds, since breeding pairs will remain in the same section of coast, even if they fail to breed successfully. Shorebird populations can usually be recorded to within a margin of error of plus or minus 10%. Breeding pairs of shorebirds are recorded on the map in the exact location at which they were recorded, using the appropriate letter code. Where more than one pair occurs too close together to mark individually on the map, they should be marked together, with the number of pairs written before the letter code, as per colonial birds.
INLAND BIRDS HOLDING TERRITORY
Conspicuous birds that hold large territories, such as raptors, can be assessed by recording their individual breeding territories. Breeding pairs patrol their own territories in search of food, making them easy to record, and with sufficient observation the actual nesting sites can usually be determined for each breeding pair. The location of each nest site should be recorded on the map using the appropriate letter code. The best time to record birds holding territory is during the chick rearing stage, when foraging activity is greatest. Accuracy is usually well within plus or minus 10%, unless specific problems in determining territorial status are encountered.
Where territories are smaller, and nest sites harder to find, numerous daily records may be necessary to determine territories. The study area should be walked twice a day, recording all bird sightings on a map, using a separate sheet for each visit. After three or four weeks the daily sightings are transferred onto one common map, with a separate map for each species. With three or four weeks of observations overlaid onto one map, territories will show up as clusters of sightings, allowing the size and number of territories to be determined, even if the actual nest sites cannot be found. The location of each territory (breeding pair) can then be marked on the survey map using the appropriate letter code. Accuracy is dependent on species type and number of recordings, but can usually be estimated from the clarity of the clusters observed.
INLAND BIRDS NOT HOLDING TERRITORY
For inland birds which do not nest in colonies, and for which territories cannot be determined, census work must rely on rough estimates of density using transect counts. The study area is crossed a number of times along set lines (transects) so that all areas and habitat types are represented. All birds observed within a set distance from the transect line are recorded in their appropriate position on the map. This distance from the transect line is called the Effective Transect Width (ETW) and is determined by species and habitat type. The ETW is the distance at which birds can be reliably sighted whilst walking the transect.
For dense habitat cover, such as woodland, a narrow ETW is required due to the difficulty of spotting birds. For open habitat, such as that found on Magdalena Island, a much wider ETW is possible because birds can be reliably sighted at a greater distance. For passerines in open habitat the ETW is set at 25 metres, so that all birds observed within 25 metres each side of the line being walked (transect) are recorded. Birds observed outside the ETW are ignored. For larger birds, such as geese, the ETW can be set at 100 metres.
The total distance walked (transect length) is recorded, and multiplied by the ETW to give the total area surveyed for each species (this will vary according to the ETW used for each species). The density is the number of individuals or pairs recorded within the survey area.
Ideally only breeding pairs should be recorded, and for geese this should be possible if sufficient time is taken, because pairs generally remain together or close by during the chick rearing period. For passerines however, it is generally impossible to determine breeding status of individual birds, and pairs are often not seen together. For this reason all birds are recorded, and the number of individuals is divided by two to give a figure for breeding pairs. This can greatly over-estimate the breeding population due to non-breeders, or under-estimate the population due to birds hidden from sight, during incubation for example.
There is no preferred time for a census of passerines, provided that it is conducted during the main breeding season, because passerines begin nesting early and often have multiple broods. Because of the nature of the census, and the difficulty in determining breeding status, the margin of error for passerines is likely to exceed plus or minus 50%. It is generally only of use in determining relative abundance.
3C. BURROWING PENGUINS
Penguins which live above ground, such as Rockhopper and Macaroni penguins, are treated in the same way as other colonial birds, as described above under section 3A. Magellanic penguins also live in loose colonies, but their nests are hidden from sight below ground in burrows, making them impossible to count in the same manner. Because the nests are in burrows, it is not possible to see how many nests are in a given area. Many burrows are unoccupied, and to assume that all burrows contain nests would greatly over-estimate the population size.
Small Magellanic penguin colonies can be counted by looking into each burrow with the aid of a flashlight to determine which burrows contain incubating birds on nests. Counts should be made immediately after the completion of egg-laying, whilst adults are incubating the eggs. The total number of occupied burrows in the colony is recorded with the aid of a tally-counter, and a spot of bright spray paint is put in front of each burrow in order to prevent double-counting or missing burrows (the paint disappears within a few days).
Burrows containing eggs but no adult are still counted as occupied nests. Because Magellanic penguins live in burrows egg losses are low, and abandoned eggs usually remain in the burrow for many days. Under-estimation due to breeding failure is therefore usually low, and the margin of error should be well within plus or minus 10% for this type of census.
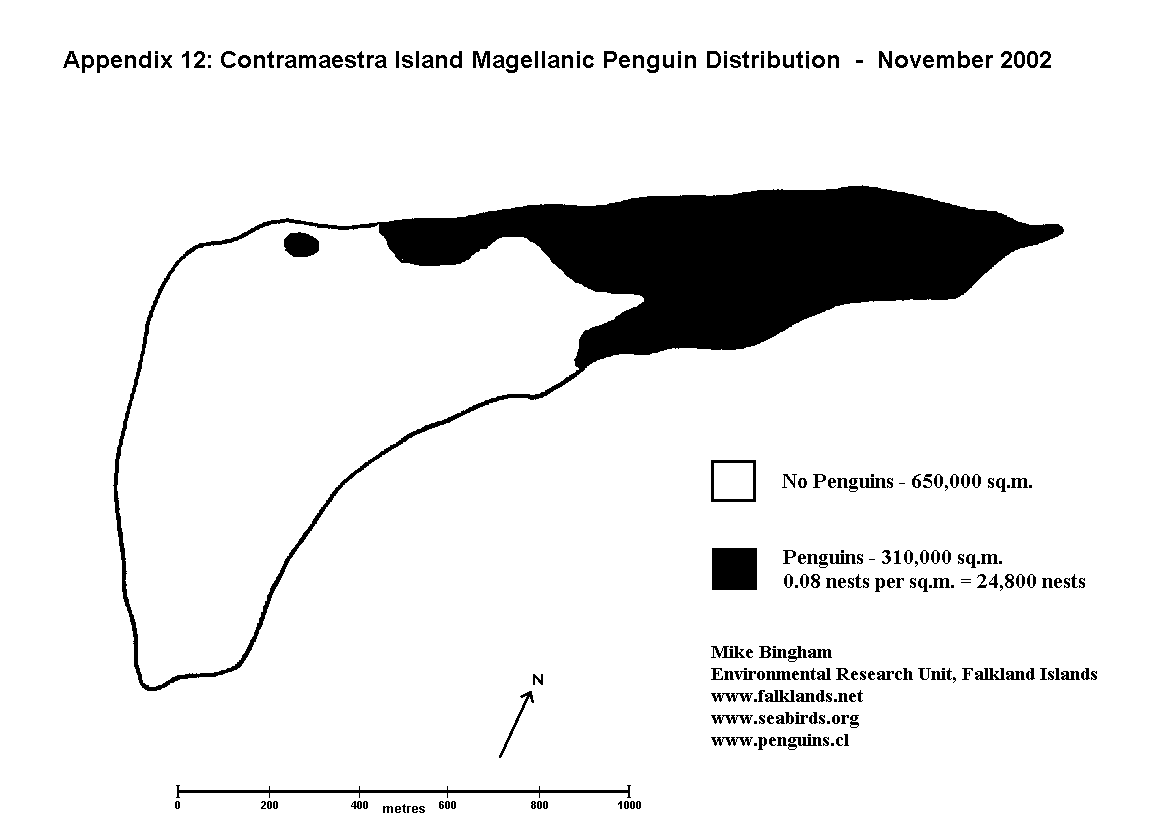
The only drawback to this methodology is that it is very time consuming, and therefore impractical for very large colonies. In such cases it is necessary to calculate the population size by mapping out the total area of the colony, and multiplying this area by the density of occupied burrows (nests/pairs) determined from study plots.
A number of study plots should be selected at random from areas within the main colony. Study plots should not cross the periphery of the colony since any area outside the colony would reduce the plot count and give a lower density reading. Plot size is determined by nesting density. For areas of moderate to high nesting density (0.05 to 0.1 nests per sq.m) the suggested plot size is 50m x 50m. For areas of nesting density below about 0.025 nests per sq.m. a plot size of 100m x 100m is recommended.
Once the study plots have been marked out, the number of occupied burrows (nests/pairs) within each study plot is counted using the methodology described above for small colonies. This gives the number of nests within a known area, allowing the mean nesting density to be calculated as nests per square metre. The total area of ground occupied by the penguin colony is then mapped out, and the area of the colony calculated from the map using a dot matrix overlay. (A dot matrix overlay is a clear acetate sheet with squares and dots used to accurately determine area from a map). The area of the colony in square metres is multiplied by the mean nesting density (nests per square metre) to give the estimated population total.
If during the above procedure it is discovered that nesting density varies by more than 25% (eg. 0.10 nests per sq.m. to 0.075 nests per sq.m.), and that the areas that lie outside this range cover greater than 10% of the total colony area, then the colony must be mapped out in greater detail according to density variation.
The colony should be mapped out to show sectors of high and low density (Appendix 8) (or high, medium and low density if the level of variation warrants it - Appendices 7 & 9). The total area covered by each density is calculated from the map using a dot matrix overlay. A number of study plots in each sector determine the mean nesting density for each sector, and this nesting density is multiplied by the appropriate area to give a separate population total for each.
EXAMPLE:
Given the criteria above, and the inherent inaccuracies of using mean density instead of direct counts, population totals obtained using the above methodology should allow for a margin of error of plus or minus 20%. Clearly direct counts as described for small colonies is preferable, but for very large colonies it is usually impractical.
4. PENGUIN MONITORING
The Baseline Survey and Population Census work described under Sections 2 and 3 above provide the basis upon which a penguin monitoring programme can be built. Such ground work is essential for the correct interpretation of any changes observed during long-term monitoring. The population census work carried out under Section 3C, when repeated annually, provides the first step of the monitoring programme.
4A. POPULATION TRENDS
One of the most important parameters of any monitoring programme is the study of population trends. Population trends indicate the overall health of a colony or population. A declining population may well indicate problems which need to be identified and rectified in order to protect the population, whilst increasing populations suggest a thriving population, even if some conflict with human activity is occurring.
In order to identify population trends it is necessary to record the population size at regular intervals, preferably every year if other factors such as breeding success or food abundance are to be recorded and related to population change. The method of recording population size each year is described under Section 3, and it is essential to ensure that the census is conducted in an identical manner each year if observed changes are to be valid. Any deviations from the stated methodology, which may be necessary because of local conditions, must be recorded in detail so that future census work can be conducted in a compatible manner.
The same permanent study plots must be used each year for determining changes in penguin population. If permanent study plots reveal annual increases or decreases in all sectors of the colony, then these observed changes can be assumed to be fairly reliable, since they are not subject to the 20% margin of error associated with turning study plot counts into population totals. Changing study plots is not recommended, since it reintroduces the 20% margin of error for each season's data, making small population changes impossible to detect.
Annual changes in area must also be considered when determining overall population change.
4B. ANNUAL BREEDING SUCCESS
Annual breeding success is the mean number of chicks reared to the point of fledging per breeding pair each year. For penguins, fledging is taken as the point at which chicks shed their mesoptile plumage and grow water-proof plumage ready to take to sea.
For penguins which breed on the surface in colonies, the number of breeding pairs within the colony is counted using methodology described in section 3A. The colony is then revisited later in the season, just prior to the chicks fledging and leaving the colony. The total number of chicks within the colony is counted, with the mean of three counts being taken as the result.
The number of chicks surviving to the point of fledging is divided by the number of breeding pairs (nests) recorded in the colony at the beginning of the breeding season. This figure is the breeding success or productivity, expressed as chicks per breeding pair. This figure may also be expressed as a percentage, where 100% is equal to 1 chick per breeding pair (nest). Provided that chicks have not already begun leaving the colony at the time of the count, productivity will be slightly over-estimated as a result of some chicks which are not at the point of fledging, and which may still die prior to fledging. However surface-breeding species are fairly uniform in development, and chick losses reduce as chicks mature, so the margin of error should be within plus or minus 10%.
It is important not to mistake juveniles, (which return to their natal colony to moult at this time of year) with moulting chicks, or an artificially high breeding success will be recorded. Careful observation of plumage will differentiate between moulting chicks and juveniles from previous seasons.
For penguins that live in burrows, such as Magellanic penguins, there are two possible ways of recording breeding success. The number of chicks surviving to fledge can be estimated from a second visit as for surface-breeding species, with the total number of chicks in any given colony or plot being divided by the number of occupied nests. However penguins living in burrows are much less uniform in development, especially when food is short, and this method can greatly over-estimate breeding success for Magellanic penguins.
Studies in the Falkland Islands have shown that chicks which receive less food take much longer to develop, causing chicks to become abandoned by the adult whilst still dependent on the adults for food, leading to high chick mortality just prior to fledging. These late developing chicks, most of which die, would be counted as successfully fledging according to the above methodology, greatly over-estimating breeding success. A much better methodology is therefore to make regular observations of egg and chick development throughout the season, right up until the point that each chick either leaves the nest to fledge or dies.
When the study plots are counted at the beginning of the breeding season, twenty occupied burrows in each plot are marked with small sticks bearing names or numbers to identify individual nests. These nests are visited on a regular basis until the chicks change their mesoptile plumage into water-proof plumage and leave the nest. Chicks disappearing prior to shedding their mesoptile plumage are presumed to have died. Chicks disappearing afterwards are presumed to have fledged (see Appendix 10).
The number of chicks fledging is divided by the number of marked burrows being observed in each study plot. This figure is the breeding success or productivity, expressed as chicks per breeding pair.
This method not only allows for accurate measurement of breeding success, but also the timing and causes of breeding failure. Abandoned eggs are opened to determine the stage of development, after it is certain that the eggs have been completely abandoned for at least two weeks. Dead chicks are removed for weighing and examination to determine causes of death. Hatching dates, development duration, and the proportion of breeding failures that result from egg losses and chick mortality can be determined.
4C. DIET AND FORAGING OBSERVATIONS
Diet and foraging behaviour are important aspects of seabird monitoring, especially when commercial fishing activities operate in the region. However many aspects of foraging behaviour are difficult to observe, except as part of a separate research programme. For a site-specific monitoring programme, observations of foraging behaviour and diet will inevitably be limited. One such limitation is the time of year during which foraging behaviour and diet can be observed.
When adults are not breeding they are not restricted to the locality of their breeding site, and are therefore difficult to observe as part of a site-specific monitoring programme. However this freedom to forage wherever food resources are most abundant means that adults find it comparatively easy to locate sufficient food, even when prey is scarce, and starvation during the winter migration is not usually a major mortality factor for adult Magellanic penguins.
During the breeding phase adults are not free to forage wherever food resources are most abundant, because their foraging range is restricted by the need to return regularly to their nest. In addition, each adult is only able to spend half the time foraging for food when brooding eggs or small chicks, as nesting duties are shared between the two parents. Chicks are totally dependent on food caught over and above what the adults require for their own metabolic needs. If adults only catch sufficient food to meet their own metabolic needs, the chicks will starve.
The usual method of determining prey composition is by stomach flushing adults returning from foraging trips. The process is not dangerous, but it is invasive, and as such it should ONLY be used when the data obtained is vital for penguin protection, and NOT just for scientific curiosity. The best place to catch adults is between the beach and their nest site. Catching adults too close to the water will allow them to escape back into the sea, whilst catching within the confines of the colony leads to excessive disturbance. It is important to ensure that only birds returning from foraging trips are caught.
Once the adult is caught, a small plastic tube (such as used in hospital for stomach-flushing infants) is passed carefully into the penguin's stomach through the open beak, taking care not to enter the wind-pipe by mistake. It is important not to apply too much pressure in order to avoid injury. Sea water is then poured into the stomach using a funnel attached to the other end of the tube. (Pump mechanisms are not recommended since it is important not to create excess pressure in the stomach). The tube is then removed, and the penguin is inverted over a bucket, so that the water in the stomach flushes out into the bucket with the stomach contents. This is repeated two or three times, until little food remains.
During the chick rearing stage it is possible to record not only prey composition, but also the quantity of food being brought back to chicks. It is therefore important to ensure that the stomach is flushed until the water is mostly free of remaining food. This may require 4 or 5 flushes. Outside of the chick-rearing phase measurements of food quantity have little significance, and it is not necessary to flush out all the stomach contents in order to determine prey composition. It is therefore better to release the bird after the majority of food appears to have been flushed.
Prior to release, the bird should be weighed, and marked with an animal-marking crayon to ensure that the same bird is not caught a second time. The stomach samples are drained and stored in jars with formaline solution or alcohol, ready for later examination. The jars should be carefully marked with date, species and location.
In the laboratory the stomach samples should be rinsed with water, and then drained and padded with cloth to remove any excess liquid. They are then weighed to determine the quantity of food retrieved (wet weight). Each sample is then divided up into its appropriate components, which are weighed individually to determine proportional dietary composition by wet weight. Fish otoliths, cephalopod beaks and crustacean carapaces (which are not easily digested) can be used to aid species identification, and to estimate proportional composition.
The number of diet samples taken, and the period of time over which samples are taken, is a balance between the need for vital data and the well-being of the birds. Whilst stomach-flushing does not cause long-term harm when carried out carefully, it is very stressful for the penguin. It is therefore important to limit such an invasive procedure to the absolute minimum.
Diet composition can also be evaluated from food dropped when adults feed chicks, and from analysis of faeces, which may contain fish otoliths, cephalopod beaks and crustacean carapaces.
For Magdalena Island, diet composition is well known from previous studies, and from ongoing collection of faeces and food scraps spilt when adults feed chicks. Stomach-flushing is therefore not considered necessary under the present monitoring regime.
Foraging duration during chick-rearing can also be recorded by marking adults in burrows that are incubating or feeding chicks. Adults in burrows can be easily marked using animal-marking crayons attached to the end of a stick which is passed down into the burrow. Each penguin should be marked around the neck and throat area where it cannot preen. Although animal-marking crayon can last several days at sea, it is important to re-apply the marking whenever it begins to fade. By marking each member of the breeding pair with a different colour, and observing the times that each penguin leaves and returns on foraging trips, it is possible to record foraging duration.
These observations are particularly important during the chick-rearing phase, when the time taken collecting food for chicks has a significant impact on chick survival. Such observations can be combined with observations of chick mortality described under section 4B.
Where financial resources permit, satellite transmitters, time-log recorders and dive-depth recorders can provide useful information on where birds forage on a daily basis, how deep they dive, how long they spend during each dive, and where they forage during the non-breeding season. However such devices increase water resistence considerably during swimming, slowing down the penguin and causing it to use more energy catching food. Adult penguins can easily cope with these problems, but such devices should NEVER be used on juveniles. Juveniles have weak muscles and lack experience at catching food. Even healthy juveniles are on the very edge of survival during the first few months after leaving the nest, and the additional drag of transmitters, recorders and flipper bands is a death sentence.
4D. ADULT & JUVENILE MORTALITY
Assuming that a colony or population is not subject to significant emigration or immigration, then population trends are a function of adult mortality, breeding success and juvenile survival. The previous sections deal with monitoring population trends and breeding success, which leaves two unknown factors in the equation: adult mortality and juvenile survival.
In a fairly self-contained population, such as the penguin population on Magdalena Island, adult mortality can be estimated by tagging large numbers of adults to see how many fail to return each year. Unfortunately because penguins have short, stubby legs, and travel through the medium of water rather than air, they cannot be ringed around the leg as for most birds. Despite extensive development, current penguin tags still cause considerable drag, reducing the penguin's ability to forage and escape predators. Existing tags also cause abrasions on the flipper, which can lead to infection. These side-effects not only cause stress to the birds, but increase mortality, which is the very factor which needs to be measured.
Juvenile survival can also be monitored through the use of tags, but the same problem exists as described above for adults. Fortunately tagging is not the only method available for estimating juvenile survival. After fledging and leaving the colony, most surviving juveniles return to their natal colony to moult each year until they are ready to breed. A rough estimate of juvenile survival can therefore be achieved by counting juveniles returning to moult each year.
Moulting juveniles are found along the beaches adjacent to the colony from January through to March. To a casual observer they can be mistaken for moulting chicks, but juveniles are easily distinguished from chicks and adults by their plumage, even during their moult. The plumage of juveniles is generally much paler than adults, but the most striking feature is the cheek area below the eye and bill, which is black in adults, but very pale in juveniles. Juveniles also lack the extensive area of pink skin above the eye and bill which is found on all adults. Juveniles differ from chicks in the facial plumage, which when huddled together is often all an observer can see.
It is worth spending time familiarising oneself with the difference in plumage between juveniles and adults / chicks before commencing the juvenile count. (NOTE: Newly moulted chicks, which have slightly different plumage, are not counted as juveniles. Juveniles must be at least one year old. Care must be taken not to mistake moulted chicks for juveniles).
Counting juveniles along the beach can be difficult and unreliable where several colonies are scattered along a long length of coastline, but for a discreet island population such as the one found on Magdalena Island, it can provide valuable data. The number of juveniles present around the coast is counted each week from end of January to end of February. These timings may differ for other locations, or for acceptional years, but the correct timing can be established from the spread of results. Counts will initially increase as a result of the daily arrival of new juveniles coming ashore to moult. Eventually a peak will be reached, and the counts will drop as juveniles begin to leave following completion of their moult. The peak figure is divided by the total number of surviving chicks estimated for the previous year, to give juveniles (year Y) per surviving chick (year Y-1).
The resulting figure is not a direct measure of the previous season's cohort, since juveniles counted do not comprise solely of chicks from the previous year. The results can initially be used only to estimate juvenile survival over the previous two or three year period, however after several years of data, statistical analysis can be employed to reveal annual changes in juvenile survival.
Despite the limitations, long-term counting of juveniles can provide invaluable data which can be used to identify years of high or low juvenile survival. Seasonal changes in juvenile survival may correspond with other observations, such as variations in breeding success, changes in prey composition, oil spills or El Niño years. Such observations can also be used to identify colonies with low juvenile survival, or to show whether years of population decline correspond to periods of low juvenile survival, helping to identify or eliminate potential causes of concern.
4E. COMPARING COLONIES
Penguin monitoring techniques described above are used to monitor the health of a particular colony or population, but they can also be used to investigate or monitor external factors which may impact certain colonies or areas within a colony. On Magdalena Island tourism is a potential cause of concern, and it is important to monitor the effects of tourism in order to ensure sustainable use of the island as a tourist resource. Human presence in the form of tourism has the potential to disturb breeding birds in a number of ways:
- Incubating birds may be frightened away allowing predators to take eggs or young.
To identify the level of disturbance, monitoring is carried out in areas that are subjected to tourism, and in control sites which are well away from tourists. Significant levels of disturbance within the study site would be evident from reduced breeding success. There may also be observed changes in predation, or the causes of egg and chick mortality. Over a longer time-scale, continued disturbance may lead to a reduction in population size.
On Magdalena Island tourists are only permitted to walk within a controlled area. Penguin burrows adjacent to this area are monitored to determine nesting density, breeding success, egg loss rates, chick mortality rates, predation and the causes of egg and chick mortality. Similar studies are conducted in other parts of the island, well away from where tourists are permitted to walk, in order to monitor any changes that may result from tourism.
Where other human activities occurring away from the breeding site are under examination, such as the impacts of commercial fishing or oil pollution, the principals are the same. Comparisons are made of study areas within the zone of human impact (eg. area that is fished or area of pollution), and control areas that are outside the zone of impact. Studies into the effects of commercial fishing or oil pollution should look for reductions in population size, breeding success, and juvenile and adult survival. Studies into the effects of commercial fishing should also look for increases in foraging range and duration, and changes in dietary composition, all of which effect chick survival.
5. REFERENCES
Bingham M. (1998) Penguins of South America and the Falkland Islands. Penguin Conservation 11(1): 8-15.
Bingham M. and Mejias E. (1999) Penguins of the Magellan Region. Scientia Marina Vol:63, Supl. 1: 485-493
Bingham M. (2002) The decline of Falklands penguins in the presence of a commercial fishing industry. Revista Chilena de Historia Natural
Boersma P.D. (1997) Magellanic penguin declines in South America. Penguin Conservation 10: 2-5
Gandini P., Boersma P.D., Frere E., Gandini M., Holik T. and Lichtschein V. (1994) Magellanic penguins affected by chronic petroleum pollution along coast of Chubut, Argentina. The Auk. 111(1): 20-27
Gandini P., Frere E. and Boersma P.D. (1998) Status and conservation of Magellanic Penguins in Patagonia, Argentina. Bird Conservation International.
Hiscock K. (1993) A manual for marine biological inventory surveys. Joint Nature Conservation Committee Report MNCR/OR/19.
6. ACKNOWLEDGEMENTS
Thanks go to the Environmental Research Unit, Corporación Nacional Forestal, ENAP, the rangers of Magdalena Island (Louis, Roberto, Domingo and Floridor), and the crews of "Melinka", "Hundy", "Don Jorge" and "Mandamiento" for logistical support, and to research assistants Nidia Mendez, Elena Mejias, Cici Legoe, Christopher Burney, Jennifer Rock and Jon Philipsborne. Special thanks to the Darwin Initiative (British Government) for providing funding for the project, and Teresa Valesco for translations.
7. APPENDICES
APPENDIX 1: TERRESTRIAL HABITAT TYPES
GRASS HEATH is dominated by long, rough grasses. On well drained sites these may adopt a tussock growth form, but on poorly drained plains they usually take on a more lax form. Where present Grass Heath supports many flowering plants, invertebrates and birds, but there was no Grass Heath recorded on Magdalena Island.
DWARF SHRUB HEATH is dominated by low growing shrubs, and is usually found on exposed, dry areas, such as hard peat overlying rocky ridges. Where present Dwarf Shrub Heath provides shelter for invertebrates, flowering plants and birds, but there was no Dwarf Shrub Heath recorded on Magdalena Island.
FELDMARK is dominated by cushion plants, often in association with ferns, dwarf shrubs and coarse grasses. It tends to be found on higher hills and exposed ridges, where the combination of thin shaley soils and exposure to wind exclude faster growing species that are less adapted to desiccation and nutrient deficiency. Where present Feldmark can provide habitat for a few specialist invertebrates and birds, but the harsh conditions and open nature excludes a diversity of species. There was no Feldmark recorded on Magdalena Island.
ROCKY OUTCROP occurs where thin soils and underlying geology result in exposed bedrock or surface stones. Where present such habitat can provide crevices for nesting birds and specialist plants, and surfaces for colonisation by lichens. There was no Rocky Outcrop recorded on Magdalena Island.
FEN is an area of tall freshwater vegetation surrounding ponds, lakes or streams. Where present Fen can provide important cover for nesting birds and invertebrates, but there was no Fen recorded on Magdalena Island.
BOG is a variable habitat comprising wet swampy areas, but there was no Bog recorded on Magdalena Island.
WOODLAND is a variable habitat comprised of trees, which needs to be further categorised according to species composition. Where present it can support a wide variety of mammals, birds, invertebrates and flora, but there was no Woodland recorded on Magdalena Island.
SAND DUNES are areas of loose or vegetated sand which form behind the littoral zone. The consolidating vegetation comprises drought and salt tolerant species able to survive in the harsh conditions. Where present Sand Dunes can provide cover for nesting birds and specialist invertebrate species, but there were no Sand Dunes recorded on Magdalena Island.
ERODED AREAS featuring exposed soil, as opposed to bedrock, caused by overgrazing, burning, physical disturbance or climatic conditions. The low-lying plains of Magdalena Island hold many eroded areas that are too small in area to be mapped. These result from a combination of low rainfall, desiccating salt-laden winds, sandy soils, and disturbance by penguins, which together prevent the establishment of vegetation. These eroded areas give rise to dust storms during strong winds. Low-lying cliffs around the island also feature eroded areas that result from land-slip and coastal erosion.
SETTLEMENTS are areas of housing or human development. Where present such areas often provide niches for specialist plants and animals, some of which are dependent on human habitation (eg. rats and mice). The only area of settlement on Isla Magdalena is the lighthouse.
GREENS are characterised by a short turf of fine grasses and flowering plants, as opposed to the tall grasses of Grass Heath. The terrestrial habitat of Magdalena Island comprises almost entirely of short grasses, mixed with drought-tolerant flowering plants and eroded areas. This is the result of low rainfall, desiccating salt-laden winds, sandy soils, and thousands of penguins that trample the ground and nutrify the soil through the deposition of guano. These Greens attract grazing geese, but a lack of natural fresh water on the island keeps the breeding population of geese low.
PASTURE is very similar to Greens, except that the grass is kept short by livestock rather than natural factors. There was no Pasture recorded on Magdalena Island.
PONDS & STREAMS There were no Ponds or Streams recorded on Magdalena Island.
APPENDIX 2: LITTORAL HABITAT TYPES
Littoral Habitats are divided into physical features and biological features.
a) Physical features:
BOULDER SHORE has stones with an average diameter of more than 300mm. Boulders provide cover for marine invertebrates avoiding desiccation at low tide, and attract feeding birds such as oystercatchers and black-crowned night herons. Boulder Shore is usually subjected to high energy waves, and does not offer safe nesting sites for birds, or suitable habitat for plants, except at the very upper reaches of the shore. There was no Boulder Shore recorded on Magdalena Island.
STONY SHORE has stones with an average diameter of between 2mm and 300mm. The shifting nature of beach stones provides a poor substrate for plants to gain a foothold, and little cover for fauna. Birds such as oystercatchers and gulls may nest on the upper reaches, but most other birds prefer sites which offer more seclusion. Stony Shore is found around the entire coast of Magdalena Island, and it is used by hundreds of gulls which nest above the high water line.
SANDY SHORE has visible grains with an average diameter of less than 2mm. Where present Sandy Shore can provide important feeding and nesting areas for waders. There was no Sandy Shore recorded on Magdalena Island.
MUDDY SHORE has soft sediment made up of grains which are too small to be visible with the naked eye. Such sediments provide rich feeding areas for waders because of the invertebrates living in the mud. Low-energy, estuarine environments are usually covered during spring tides, precluding nesting or the establishment of terrestrial vegetation. There was no Muddy Shore recorded on Magdalena Island.
ROCKY SHORE is made up of exposed bedrock which provides secure attachment for marine invertebrates such as mussels and limpets, and for marine algaes which in turn support other marine creatures. Rockpools also tend to be numerous at low tide, trapping small fish and marine creatures. This wealth of marine life provides rich feeding for birds such as oystercatchers, black-crowned night herons and gulls. The high energy waves prevent nesting, or the establishment of terrestrial plants, except in the upper reaches. There was no Rocky Shore recorded on Magdalena Island.
CLIFFS are steep inclines of underlying rock that exceed 8m in height, and there are several areas of cliff around Magdalena Island. Cliffs provide unsuitable feeding or nesting areas, except for a few seabirds such as gulls and rock shags. Cliffs around Magdalena Island are made up of soft sedimentary rock, which Magellanic penguins use for burrows wherever they can reach. These soft sedimentary rocks are subject to coastal erosion and landslip, which prevents the establishment of cliff flora.
b) Biological features:
GREEN ALGAE is where significant amounts of green algae (Ulva sp.) is found. Sea lettuce tends to grow around the mean tide level, where it provides a valuable food resource for shorebirds such as kelp geese. Although some green alga is present, no significant areas were recorded around Magdalena Island.
KELP BEDS are areas where kelps, such as giant kelp (Macrocystis pyrifera) and tree kelp (Lessonia sp.) can be found in the sub-littoral zone. Kelp beds provide an important ecological niche supporting small fish and invertebrates, making them important feeding areas for seabirds such as cormorants. Kelp beds were recorded around the entire coast of Magdalena Island.
MUSSEL BEDS are areas where large numbers of mussels are present. Mussel beds provide an important food resource for birds such as oystercatchers and gulls, especially during the winter when other food is scarce. One significant area of mussels was found along the northern coast of Magdalena Island. . .
. .
. .
. .
. .
. .
. .
. .
. .
.
. .
Seabird Monitoring Instruction Manual
2. BASELINE SURVEY
3. POPULATION CENSUS
4. PENGUIN MONITORING
5. REFERENCES
6. ACKNOWLEDGEMENTS
7. APPENDICES
High Density: Area = 492,090 sq.m Mean Density = 0.098 nests/sq.m.
TOTAL = 48,225 breeding pairs (occupied nests)
Medium Density: Area = 115,223 sq.m Mean Density = 0.077 nests/sq.m.
TOTAL = 8,872 breeding pairs (occupied nests)
Low Density: Area = 39,054 sq.m Mean Density = 0.050 nests/sq.m.
TOTAL = 1,953 breeding pairs (occupied nests)
TOTAL FOR COLONY = 59,050 breeding pairs
- Raised metabolic rates brought on by stress may lead to greater food requirement.
- Natural behaviour, such as courtship or the feeding of young, may be disrupted.
- Adults could be scared away completely, causing them to abandon eggs or young.
- Severe disturbance could lead to adults or young being killed or injured.
- Birds living in burrows may be killed if the burrow collapses under human weight.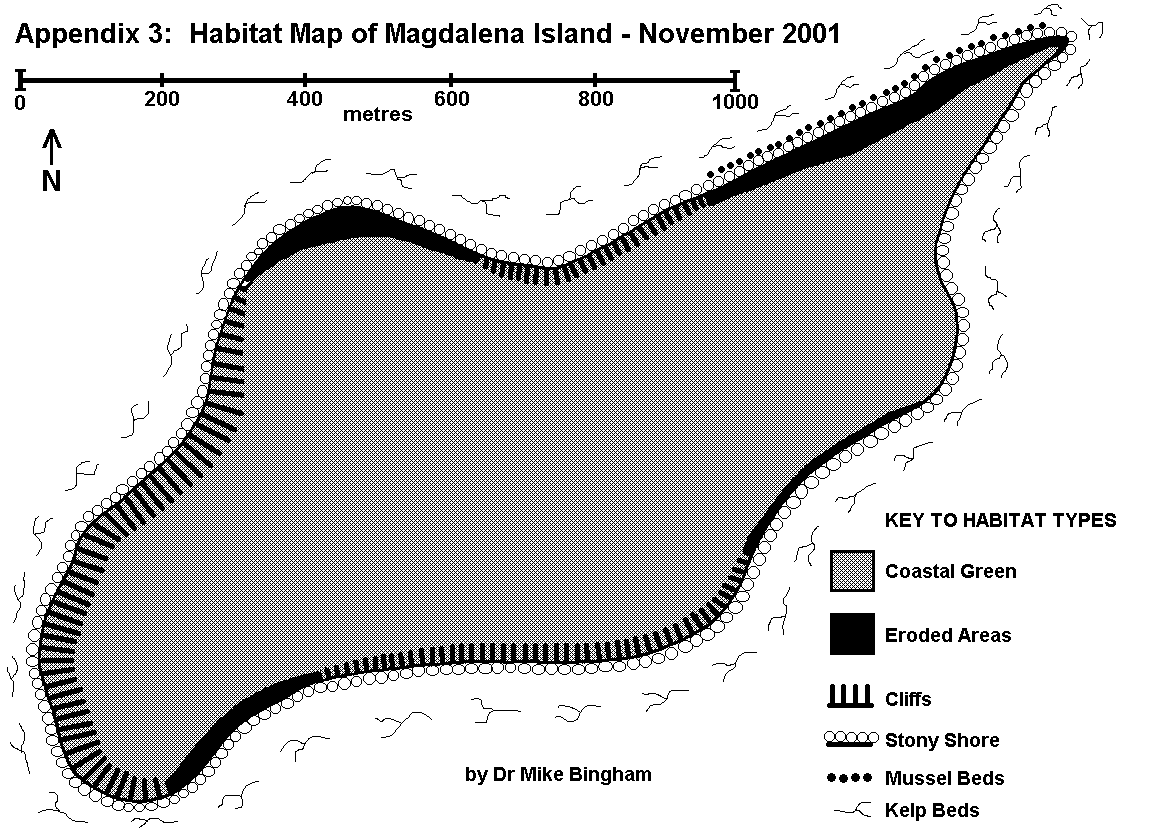



Mike Bingham, © 2022